Pyrolysis and Combustion of Wood
The combustion of wood is a complex process depending on many different factors such as density, moisture content, thermal conductivity, heat capacity, permeability and actual thickness of the wood. Below is a brief summary of how timber reacts when exposed to fire.
The process is most aptly described in Browne (1958), Wood does not burn directly; it first undergoes thermal decomposition known as pyrolysis. Under appropriate conditions the products (volatile, combustible gases and vapours) of this can be set afire and if the wood retains enough of the heat of combustion, a self-sustaining reaction can occur until only inorganic products are left such as ash.
Usually this occurs by introducing a pilot flame, which then will ignite with the gaseous products. Without this pilot, heat flux would have to be doubled to allow the wood to ignite spontaneously. Wood may be said to be burn directly if its temperature is raised to the point where pyrolysis and combustion are practically simultaneous.
The main concept of flameproofing wood and other cellulosic materials is by changing the pyrolysis mechanism from fast to slow pyrolysis by the addition of chemical coatings. This results in the production of more charcoal at the expense of more flammable products, this is desired as described both below and in the charring section as it insulates the rest of the wood. (30)
The process is most aptly described in Browne (1958), Wood does not burn directly; it first undergoes thermal decomposition known as pyrolysis. Under appropriate conditions the products (volatile, combustible gases and vapours) of this can be set afire and if the wood retains enough of the heat of combustion, a self-sustaining reaction can occur until only inorganic products are left such as ash.
Usually this occurs by introducing a pilot flame, which then will ignite with the gaseous products. Without this pilot, heat flux would have to be doubled to allow the wood to ignite spontaneously. Wood may be said to be burn directly if its temperature is raised to the point where pyrolysis and combustion are practically simultaneous.
The main concept of flameproofing wood and other cellulosic materials is by changing the pyrolysis mechanism from fast to slow pyrolysis by the addition of chemical coatings. This results in the production of more charcoal at the expense of more flammable products, this is desired as described both below and in the charring section as it insulates the rest of the wood. (30)
Combustion of Wood with respect to heat release rates
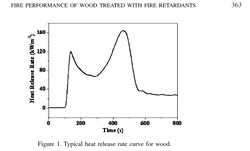
Marney et al (2008) (17) explain the process of wood combustion with reference to heat release rates.
“After an initial heating induction period, a sufficient amount of pyrolysis volatile gases are evolved to allow ignition by an external spark igniter. This is the commencement of combustion (i.e. reaction between oxygen and volatile gases under the influence of heating). The heat generated by these exothermic (combustion) reactions along with the externally applied heat sustains the pyrolysis of the wood, thus releasing more volatiles for ongoing sustenance of the flaming combustion process. This corresponds with the first peak in the HRR curve. After the initial release of volatiles, an insulating char layer forms, which makes heat transfer more difficult, thus slowing the pyrolysis process. This corresponds with the dip in the HRR curve and as the wood is sufficiently thick, the HRR reaches a more or less steady state. The second peak in the HRR curve results from the char layer breaking down and contracting, thus producing small cracks on the surface. These cracks facilitate the escape of volatiles that combust and result in the observed increase in HRR. After the volatiles have been exhausted, flaming combustion ends, leaving a solid char residue and reduced HRR.” Section 3.1 pg 362
“After an initial heating induction period, a sufficient amount of pyrolysis volatile gases are evolved to allow ignition by an external spark igniter. This is the commencement of combustion (i.e. reaction between oxygen and volatile gases under the influence of heating). The heat generated by these exothermic (combustion) reactions along with the externally applied heat sustains the pyrolysis of the wood, thus releasing more volatiles for ongoing sustenance of the flaming combustion process. This corresponds with the first peak in the HRR curve. After the initial release of volatiles, an insulating char layer forms, which makes heat transfer more difficult, thus slowing the pyrolysis process. This corresponds with the dip in the HRR curve and as the wood is sufficiently thick, the HRR reaches a more or less steady state. The second peak in the HRR curve results from the char layer breaking down and contracting, thus producing small cracks on the surface. These cracks facilitate the escape of volatiles that combust and result in the observed increase in HRR. After the volatiles have been exhausted, flaming combustion ends, leaving a solid char residue and reduced HRR.” Section 3.1 pg 362
Pyrolysis
‘In the area of fire safety, the process of wood pyrolysis is of fundamental importance in structural fires, where structural timber members undergo pyrolysis and subsequent combustion.’ (Reszka 2008 pg1)(32).
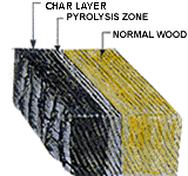
The actual Pyrolysis Zone is an area of heated wood usually with a temperature above 200°C undergoing irreversible chemical decomposition accompanied by discolouration and loss of mass (which reduces the effective cross section and therefore the member's ability to resist mechanical loads), caused solely by increased heat exposure.
Onset of pyrolytic behaviour usually occurs at 200°C (20)(24)(26) or at about 10 kW/m² (32) in terms of the heat flux (heat release rate per per unit area) exposed to the heated side.
(34)
Between 200 and 300°C some wood components undergo significant pyrolysis with both carbon monoxide and tar being produced. Dehydration reactions around 200°C are responsible for the pyrolysis of hemicellulose and lignin and results in a high char yield (White & Dietenberger (2008)) (26).
