Fire Science: An Overview
The fire science behind the field of timber framed structures in fire is a large and heavily investigated one with many different entities being both responsible and interested in it. There is a lot of information already known about the subject, the most of important and fundamental of which is summarized in the Pyrolysis and Charring sections for your convenience and interest.
Below is some basic fire science that will hopefully help you understand the overall science involved in the combustion of wood.
Below is some basic fire science that will hopefully help you understand the overall science involved in the combustion of wood.
Basic Fire Chemistry
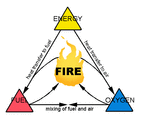
- For a fire to occur three basic components are required;
- 1. fuel (in this case timber)
- 2.an oxidizer (usually oxygen)
- 3. energy (heat).
Therefore for timber (wood) to ignite it needs to be in contact with air and a heat source needs to be applied to increase the temperature of the wood up until a point were ignition occurs. This usually occurs due to the fuel having too much molecular energy due to the heat source and therefore seeks to 'offload' this by creating an exothermic reaction with the oxidizer, which is what is known as combustion or simply 'fire'.
Fire Retardant Science
Fire/Flame Retardants work by preventing or interfering with the combustion process shown above (15). However they do not inherently make any significant contribution to increasing the fire resistance of a structure (10)(40).
Several definitions of flame retardance are offered below but the main principle is that they do not stop the fuel from burning but they inhibit the fire's growth by curtailing either the flame spread rate, time to ignition or heat release rate or all three (36)(37)(40).
Greene (1995) on page 1 , "Flame-retarded materials can be formulated to be more resistant to ignition than non-flame retarded materials or to have slower rates of flame spread in a major fire... however the flame-retarded article will ultimately burn." (15)
Horrocks (2001) 1.1.5 on page 9, "not the fact that their products burn how to render them less likely to ignite, and if they are ignited, burn less efficiently. This phenomenon is termed 'flame retardance'." (18)
Retardant Chemistry
Retardant mechanisms are described more in the Retardant section, however below is a brief introduction into how some retardants work.
1. Dilution - "Reducing the total quantity of combustible material improves overall flame retardance." (15)
2. Generation of Non-combustible Gas - "Some materials decompose when heated generating non-combustible gases. These lower the oxygen concentration at the front of the flame." (15)
3.Gas-Phase, Free-Radical Inhibition - de-activate free radicals in the vapour phase (i.e. retardant reacts with oxygen instead of free radicals), which results in fewer reactions with oxidizer, which results in less heat being generated and the overall combustion process is slowed (15). Bromine compounds are very effective at removing OH* and H* radicals which inhibits flame growth/oxidation.(25)
4. Solid-Phase Char Formation - "Several flame retardants form 'insulating or minimally combustible chars' when exposed to heat. This char reduces volatilization of active fragments and absorbs and dissipates heat." (15) Intumescent Paints are a good example of this process, they increase the char production at the expense of flammable, volatile products of pyrolysis, thus suppressing combustibility (24).
Several definitions of flame retardance are offered below but the main principle is that they do not stop the fuel from burning but they inhibit the fire's growth by curtailing either the flame spread rate, time to ignition or heat release rate or all three (36)(37)(40).
Greene (1995) on page 1 , "Flame-retarded materials can be formulated to be more resistant to ignition than non-flame retarded materials or to have slower rates of flame spread in a major fire... however the flame-retarded article will ultimately burn." (15)
Horrocks (2001) 1.1.5 on page 9, "not the fact that their products burn how to render them less likely to ignite, and if they are ignited, burn less efficiently. This phenomenon is termed 'flame retardance'." (18)
Retardant Chemistry
Retardant mechanisms are described more in the Retardant section, however below is a brief introduction into how some retardants work.
1. Dilution - "Reducing the total quantity of combustible material improves overall flame retardance." (15)
2. Generation of Non-combustible Gas - "Some materials decompose when heated generating non-combustible gases. These lower the oxygen concentration at the front of the flame." (15)
3.Gas-Phase, Free-Radical Inhibition - de-activate free radicals in the vapour phase (i.e. retardant reacts with oxygen instead of free radicals), which results in fewer reactions with oxidizer, which results in less heat being generated and the overall combustion process is slowed (15). Bromine compounds are very effective at removing OH* and H* radicals which inhibits flame growth/oxidation.(25)
4. Solid-Phase Char Formation - "Several flame retardants form 'insulating or minimally combustible chars' when exposed to heat. This char reduces volatilization of active fragments and absorbs and dissipates heat." (15) Intumescent Paints are a good example of this process, they increase the char production at the expense of flammable, volatile products of pyrolysis, thus suppressing combustibility (24).
